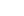
Principle of vacuum nitriding treatment
Gas nitriding generally uses anhydrous ammonia (or ammonia + hydrogen, or ammonia + nitrogen) as the nitrogen supply medium. The entire nitriding process can be divided into three stages.
(1) Decomposition of ammonia
Ammonia is a very unstable gas that is easy to decompose under certain conditions. Its decomposition rate increases with the increase of temperature, in the temperature range of 400~600 °C, its natural decomposition rate can tend to all decompose, and its decomposition reaction is as follows:
2NH3=====2[N]+6[H] The reactive nitrogen atom decomposed in ammonia is a new ecological nitrogen atom, with great chemical activity, partially absorbed by the surface of the workpiece, and then diffused from the surface to the inside, and the remaining [N]
quickly formedThe synthetic molecular N2 is discharged from the exhaust gas together with H2, so the ammonia decomposition formula is actually: 2NH3======2[N]+6H=====3H2+N2
In order for nitriding to continue continuously
, ammonia needs to be continuously input to continuously produce active nitrogen atoms
(2) The surface of the steel absorbs nitrogen atoms
After the reactive nitrogen atom is absorbed by the surface of the steel, it penetrates deep into the ferrite to form a ferrite with high nitrogen content, and after supersaturation, it forms nitrides.
(3) Diffusion After the surface of the
steel absorbs nitrogen atoms, there is a nitrogen concentration gradient on the surface and inner layer, which promotes the diffusion of nitrogen atoms from the surface inward to form a nitriding layer of a certain thickness.
At the nitriding temperature, the active nitrogen atoms in the adsorption layer move to the inside of the metal lattice, and the voids left are quickly filled by the nitrogen atoms of the adsorption layer, so that the reactive nitrogen atoms on the metal surface are always kept continuously infiltrating. Therefore, the diffusion process is as follows.
(<>) Continuously input nitrogen-containing gas into the furnace;
Migration of ammonia molecules to metal surfaces;
Ammonia molecules are adsorbed by metal surfaces;
Ammonia is continuously decomposed at the phase interface to form nitrogen atoms and hydrogen atoms;
Absorb the remaining active atoms and composite components and continuously discharge them from the furnace;
The nitrogen atoms adsorbed on the surface are dissolved in γ-Fe and α-Fe.
(<>) Nitrogen atoms diffuse inward from the metal surface and produce a certain concentration gradient.
(<>) When nitrogen exceeds the solubility in α-Fe, the surface layer begins to form nitrides.
(<>) Nitride grows along the vertical and parallel directions of the metal surface.
(<>) The surface forms γ phase and ε phase in turn.
(<>) The nitriding layer continues to thicken.
(<>) Nitrogen diffuses from the nitride layer to the interior of the metal.
There are many factors that affect the above basic performance, such as temperature, time, pressure, medium composition (or nitrogen potential), and part steel composition and structure. The gas nitriding process is to reasonably control these influencing factors and obtain a satisfactory nitriding layer.